Two human genes, BRCA1 and BRCA2 (BRCA1/2), produce proteins that block the growth of cancer, such as breast or ovarian cancer. These proteins ensure the stability of each cell’s genetic material and help to repair damaged DNA. A mutation in either BRCA results in these proteins not functioning correctly. Specifically, DNA damage may not be repaired effectively, which can lead to cancer.1
In the general US population, approximately 12% of women will have breast cancer at some point during their lives.2 Furthermore, a large study conducted in 2017 estimated that approximately 72% of women who inherit a BRCA1 mutation and approximately 69% of women who inherit a harmful BRCA2 mutation will have breast cancer by the age of 80 years.3
In 2018, the US Food and Drug Administration (FDA) expanded the indications for the first poly (ADP-ribose) polymerase (PARP) inhibitor, olaparib (Lynparza), to become the first PARP inhibitor approved for the treatment of women with advanced breast cancer and a deleterious or suspected deleterious germline BRCA mutation, as detected by BRACAnalysis CDx test.4 The other 2 PARP inhibitors approved by the FDA, rucaparib (Rubraca) and niraparib (Zejula), are approved for other types of tumors but not for breast cancer. Until recently, these were the 3 PARP inhibitors approved by the FDA.5
Talazoparib Approved for Breast Cancer with Germline BRCA Mutation
On October 16, 2018, the FDA approved talazoparib (Talzenna; Pfizer), an oral PARP inhibitor, for the treatment of adults with deleterious or suspected deleterious germline BRCA mutation–positive, HER2-negative locally advanced or metastatic breast cancer.6 The presence of a germline BRCA mutation must be identified by the FDA-approved companion diagnostic BRACAnalysis CDx test.6 With this approval, talazoparib becomes the fourth PARP inhibitor available and the second that is FDA approved for the treatment of patients with germline BRCA mutation–positive advanced breast cancer and the fourth PARP inhibitor approved in the United States.5
Mechanism of Action
Talazoparib inhibits PARP enzymes, which play a role in DNA repair. Studies conducted in cancer-cell lines with defects in DNA repair genes, including BRCA1 and BRCA2, show that talazoparib-induced cytotoxicity may involve blocking PARP enzymatic activity and increased formation of PARP–DNA complexes. This action results in DNA damage, decreased cell proliferation, and apoptosis.7
Dosing and Administration
The recommended starting dose of talazoparib is a 1-mg capsule taken orally once daily until disease progression or until unacceptable toxicity. Capsules can be taken with or without food. A smaller, 0.25-mg capsule is available when dose reduction is needed.7
Talazoparib capsules should be swallowed whole. Patients who vomit or miss a dose should not take an additional dose, but should take the next prescribed dose at the usual time.7
Patients should be considered for talazoparib based on the presence of a deleterious or a suspected deleterious germline BRCA mutation using the FDA-approved companion diagnostic test BRACAnalysis CDx.7
Pivotal Clinical Trial: EMBRACA
The efficacy of talazoparib was evaluated in the phase 3 clinical trial EMBRACA.8 In this open-label study, 431 patients with germline BRCA mutation–positive, HER2-negative locally advanced or metastatic breast cancer were randomized to talazoparib 1 mg once daily (N = 287) or to the physician’s choice of single-agent chemotherapy with capecitabine, eribulin, gemcitabine, or vinorelbine (N = 144).8 All patients had to have received no more than 3 previous cytotoxic chemotherapy regimens for locally advanced or metastatic breast cancer. Unless contraindicated, patients had to have received an anthracycline and/or a taxane in the neoadjuvant, adjuvant, and/or metastatic treatment setting.8
The majority (approximately 98%) of patients had an Eastern Cooperative Oncology Group performance status of 0 or 1. More than half (54.7%) of the patients who received talazoparib had hormone receptor–positive breast cancer. Nearly half (45.3%) of those in the talazoparib arm had triple-negative breast cancer versus 41.7% in the chemotherapy arm. In total, 15% and 13.9% of the patients, respectively, had a history of central nervous system (CNS) metastases.8
Overall, 91% of patients in the talazoparib arm received previous taxane therapy, and 85% received an anthracycline. In addition, 16% of patients in the chemotherapy arm and 20.8% of patients in the talazoparib arm received platinum. The primary efficacy end point was progression-free survival (PFS) based on Response Evaluation Criteria in Solid Tumors and a blinded independent review.8
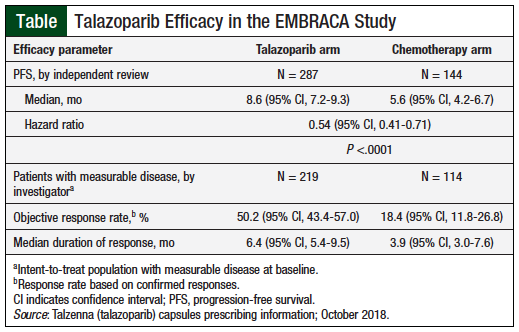
After a median follow-up of 11.2 months, the PFS was 8.6 months with talazoparib versus 5.6 months with chemotherapy (hazard ratio [HR] for disease progression or death, 0.54; 95% confidence interval [CI], 0.41-0.71; P <.001). Consistent PFS results were observed regardless of line of therapy, triple-negative breast cancer status, and CNS metastases.8
After 1 year of treatment, 37% of patients in the talazoparib arm and 20% of those receiving chemotherapy had no disease progression. By the time of the primary analysis, 108 patients had died in the talazoparib arm versus 55 in the chemotherapy arm. The median overall survival during an interim analysis was 22.3 months (95% CI, 18.1-26.2) versus 19.5 months (95% CI, 16.3-22.4), respectively (HR for death, 0.76; P = .11).8 The Table highlights the efficacy data.7
Adverse Events
The safety profile of talazoparib is based on data from 286 patients in the EMBRACA study. The median duration of treatment with talazoparib in this study was 6.1 months.7 The most common (≥20%) adverse reactions of any grade reported with talazoparib were fatigue (62%), anemia (53%), nausea (49%), neutropenia (35%), headache (33%), thrombocytopenia (27%), vomiting (25%), alopecia (25%), diarrhea (25%), and decreased appetite (21%).7
Dosing interruption because of an adverse reaction occurred in 65% of patients who received talazoparib. Dosing reduction because of any cause occurred in 53% of patients who received talazoparib. Overall, 5% of patients permanently discontinued talazoparib because of adverse reactions.7
Talazoparib has no contraindications.7
Drug Interactions
The coadministration of talazoparib with specific P-glycoprotein (gp) inhibitors (ie, amiodarone, carvedilol, clarithromycin, itraconazole, and verapamil) increases exposure to talazoparib. If coadministration of talazoparib and these P-gp inhibitors cannot be avoided, the talazoparib dose should be reduced. When the P-gp inhibitor is discontinued, the talazoparib dose can be increased (after 3 to 5 half-lives of the P-gp inhibitor), to the dose that was used before starting the P-gp inhibitor.7
The coadministration of breast cancer resistance protein inhibitors with talazoparib may increase talazoparib exposure. Patients taking both treatments should be monitored for increased risk for adverse reactions.7
Use in Specific Populations
Talazoparib can cause fetal harm. Females of reproductive potential should use effective contraception during treatment and for at least 7 months after the last dose of talazoparib. Males of reproductive potential should use effective contraception during treatment and for at least 4 months after the last dose of talazoparib.7
Women should not breastfeed during treatment with talazoparib and for at least 1 month after the final dose.7
The safety and effectiveness of talazoparib have not been established in children.7
No differences in the safety or effectiveness of talazoparib were observed between older patients (aged ≥65 years) and younger patients who participated in clinical trials.7
The recommended dose of talazoparib should be reduced in patients with moderate renal impairment (creatinine clearance levels between 30 and 59 mL/min). Talazoparib has not been studied in patients with severe renal impairment or in patients who require hemodialysis.7
Talazoparib has not been studied in patients with moderate or severe hepatic impairment.7
Warnings and Precautions
Myelodysplastic syndrome and acute myeloid leukemia have been reported in 2 of 584 (0.3%) patients with solid tumors who received talazoparib in clinical studies. Myelosuppression (ie, anemia, leukopenia, neutropenia, and/or thrombocytopenia) has been reported in patients who received talazoparib. Complete blood counts should be evaluated at baseline and monthly thereafter with the use of talazoparib.7
Conclusion
Talazoparib is the fourth PARP inhibitor approved by the FDA and the second approved for the treatment of women with HER2-negative advanced breast cancer and germline BRCA mutations. Talazoparib has demonstrated superior PFS compared with chemotherapy in this patient population and a numerically better overall survival based on an interim analysis. Ongoing clinical trials are evaluating the efficacy and safety of talazoparib in other types of advanced breast cancer, ovarian cancer, and other solid tumors.
References
- National Cancer Institute. BRCA mutations: cancer risk and genetic testing. www.cancer.gov/about-cancer/causes-prevention/genetics/brca-fact-sheet. Accessed January 20, 2019.
- American Cancer Society. How common is breast cancer? Revised January 8, 2019. www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html/. Accessed January 20, 2019.
- Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402-2416.
- US Food and Drug Administration. FDA approves olaparib for germline BRCA-mutated metastatic breast cancer. January 12, 2018. Updated October 3, 2018. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm592357.htm. Accessed January 17, 2019.
- Honey K. FDA approves a new PARP inhibitor for BRCA-mutant breast cancer. Cancer Research Catalyst. October 17, 2018. https://blog.aacr.org/fda-approves-a-new-parp-inhibitor-for-brca-mutant-breast-cancer/. Accessed April 5, 2019.
- US Food and Drug Administration. FDA approves talazoparib for gBRCAm HER2-negative locally advanced or metastatic breast cancer. October 16, 2018. Updated December 17, 2018. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm623540.htm. Accessed January 17, 2019.
- Talzenna (talazoparib) capsules, for oral use [prescribing information]. New York, NY: Pfizer; October 2018.
- Litton JK, Rugo HS, Ettl J, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379:753-763.