Gene mutations or rearrangements in the tropomyosin receptor kinase (TRK) family of receptor tyrosine kinases are emerging as an important driver of cancer-cell growth in a wide range of cancers.1-3 Research has shown that neurotrophic receptor tyrosine kinase (NTRK) genes, which encode for TRK proteins, can fuse abnormally to other genes and enhance cell signals that support tumor growth.3 NTRK gene fusions are found in a variety of tumor types, including soft-tissue sarcoma, salivary gland cancer, infantile fibrosarcoma, thyroid cancer, and lung cancer.3
In addition to next-generation sequencing (NGS) methods, novel compounds are being developed to block TRK proteins selectively and stop cancer-cell growth. Multiple clinical trials of small molecules that inhibit TRK are underway in various cancer types.2
Larotrectinib Approved for Solid Tumors with NTRK Mutation
On November 26, 2018, the US Food and Drug Administration (FDA) approved larotrectinib (Vitrakvi; Loxo Oncology), the first drug approved for the treatment of adults and pediatric patients with solid tumors that have an NTRK gene fusion without a known acquired resistance mutation, are metastatic or where surgical resection is likely to result in severe morbidity, and have no satisfactory alternative treatments or that have progressed after treatment.4,5 Larotrectinib received accelerated approval based on overall response rate and duration of response data.4-6 Further clinical trials are required to confirm the clinical benefit of larotrectinib.4,5
Commenting on this approval, FDA Commissioner Scott Gottlieb, MD, said, “Today’s approval marks another step in an important shift toward treating cancers based on their tumor genetics rather than their site of origin in the body.”4
Mechanism of Action
Larotrectinib is an inhibitor of TRK, including TRKA, TRKB, and TRKC.5 These kinases are encoded by the genes NTRK1, NTRK2, and NTRK3. Using in vitro and in vivo tumor models, larotrectinib demonstrated antitumor activity in cells with constitutive activation of TRK proteins that resulted from gene fusions or the deletion of a protein regulatory domain, and in cells with TRK protein overexpression.5 Point mutations in the TRKC kinase domain (ie, G623R, G696A, and F617L) result in resistance to larotrectinib.5
Dosing and Administration
Patients should be selected for the consideration of larotrectinib treatment based on the presence of NTRK gene fusion in the tumor specimens. Currently no FDA-approved test is available for the detection of NTRK gene fusion.4,5 In clinical trials, the identification of positive NTRK gene fusion status was determined prospectively in local laboratories using NGS or fluorescence in situ hybridization testing.5,6
The recommended starting dose of larotrectinib in adults and young patients with a body surface area of ≥1 m2 is 100 mg orally twice daily, with or without food, until disease progression or unacceptable toxicity.5 For pediatric patients whose body surface area is <1 m2, the recommended starting dose of larotrectinib is <100 mg/m2 orally twice daily.5
Larotrectinib is available in a capsule form and as an oral solution (20 mg/mL). These dosage forms can be used interchangeably. Larotrectinib capsules should be swallowed whole and should not be chewed or crushed.5
Clinical Trials
The efficacy of larotrectinib was evaluated in adults and pediatric patients with unresectable or metastatic solid tumors associated with an NTRK gene fusion based on 3 clinical trials.5,6 The 3 clinical trials of larotrectinib included a dose-finding study in adults (LOXO-TRK-14001; N = 70); a dose-finding study in pediatric patients (SCOUT; N = 43); and a single-arm study (NAVIGATE; N = 63).5
The most common tumors of patients in these 3 studies, in order of decreasing frequency, were soft-tissue sarcoma (16%), salivary gland tumors (11%), lung cancer (10%), thyroid cancer (9%), colon cancer (8%), infantile fibrosarcoma (8%), primary central nervous system cancer (7%), and melanoma (5%).5 All patients had disease progression after systemic therapy, if available, or required surgery associated with significant morbidity for locally advanced cancer.5,6
The adults in these clinical trials received larotrectinib 100 mg orally twice daily; the pediatric patients (aged ≤18 years) received larotrectinib at a dose of 100 mg/m2, with a maximum dose of 100 mg orally twice daily. Treatment continued until unacceptable toxicity or until disease progression.5,6
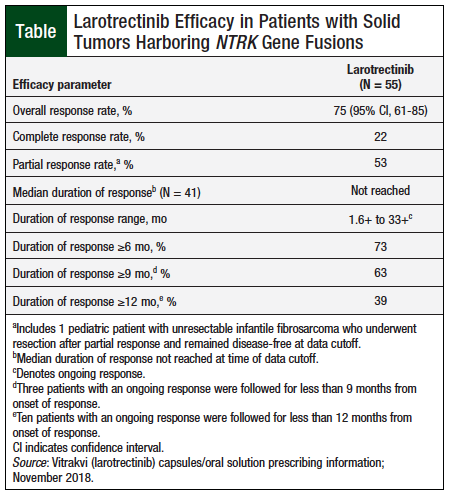
An assessment of larotrectinib’s efficacy was based on the first 55 patients enrolled in the 3 studies. Efficacy outcome measures included overall response rate and duration of response, as determined by a blinded Independent Review Committee using the Response Evaluation Criteria in Solid Tumors (RECIST) guideline, version 1.1.5,6
The key efficacy findings for larotrectinib are summarized in the Table.5 Additional efficacy data, by tumor type, are provided in the prescribing information of larotrectinib. The largest patient cohorts were salivary gland tumors (N = 12) and soft-tissue sarcoma (N = 11), and the overall response rates for these populations were 83% and 91%, respectively.5
Adverse Events
The safety profile of larotrectinib is based on data from 176 patients, including 70 (40%) patients who were exposed to larotrectinib for more than 6 months and 35 (20%) patients who were exposed for more than 1 year.5
The most common (≥20%) adverse reactions observed among patients who received larotrectinib, in order of decreasing frequency, were fatigue, nausea, dizziness, vomiting, anemia, increased aspartate transaminase, cough, increased alanine transaminase, constipation, and diarrhea.5 Grade 3 or 4 adverse reactions occurred in 51% of patients.5 The most common (≥2%) serious adverse events associated with larotrectinib were pyrexia, diarrhea, sepsis, abdominal pain, dehydration, cellulitis, and vomiting.5
Grade 3 neurologic adverse reactions included delirium (2%), dysarthria (1%), dizziness (1%), gait disturbance (1%), and paresthesia (1%); grade 4 encephalopathy (0.6%) occurred in 1 patient.5
Adverse reactions that led to treatment interruption or reduction of the larotrectinib dose occurred in 37% of patients, and 13% of patients permanently discontinued larotrectinib because of adverse reactions.5
Larotrectinib has no contraindications.5
Drug Interactions
Larotrectinib should not be coadministered with strong cytochrome (CY) P3A4 inhibitors. If coadministration cannot be avoided, the dose of larotrectinib should be reduced by 50%. After the CYP3A4 inhibitor has been discontinued for 3 to 5 elimination half-lives, the dose of larotrectinib that was taken before the initiation of the CYP3A4 inhibitor can be resumed.5
Larotrectinib should also not be coadministered with strong CYP3A4 inducers. If coadministration cannot be avoided, the dose of larotrectinib should be doubled. After the CYP3A4 inducer has been discontinued for 3 to 5 elimination half-lives, the dose of larotrectinib that was taken before the initiation of the CYP3A4 inducer can be resumed.5
Use in Specific Populations
Larotrectinib can cause fetal harm. Females of reproductive potential should use effective contraception during treatment and for at least 1 week after the last dose of larotrectinib. Males of reproductive potential should use effective contraception during treatment and for at least 1 week after the last larotrectinib dose.5
Women should not breastfeed during treatment with larotrectinib and for at least 1 week after the final dose.5
Because of the small number of adults and pediatric patients who have received larotrectinib in clinical trials, the single-arm design of these studies, and other confounding factors (ie, differences in susceptibility to infections between adults and pediatric patients), it cannot be determined whether differences in the incidence of adverse reactions to larotrectinib are related to patient age or to other factors.5 Severe adverse reactions that occurred more frequently (≥5% increase in per-patient incidence) in children than in adults were increased weight (11% vs 2%) and neutropenia (20% vs 2%).5 The pharmacokinetics of larotrectinib in children and adults were similar.5
Warnings and Precautions
Neurotoxicity has been reported with larotrectinib treatment. The majority of neurologic adverse reactions occurred within the first 3 months of treatment (range, 1 day-2.2 years).5
Modification of the larotrectinib dose was required in patients who had dizziness (3%), gait disturbance (1%), delirium (1%), memory impairment (1%), and tremor (1%).5 Larotrectinib treatment should be withheld or permanently discontinued based on the severity of neurotoxicity.5
Liver toxicity, including increased transaminases, has been reported with larotrectinib treatment. Patients receiving larotrectinib should undergo liver test monitoring, including alanine transaminase and aspartate transaminase, every 2 weeks during the first month of treatment, then monthly thereafter, and as clinically indicated.5
Conclusion
Larotrectinib, a novel oral inhibitor of TRK, is the first therapy approved for the treatment of adults and pediatric patients with solid tumors associated with an identified NTRK gene fusion. It is approved for patients with solid tumors and this biomarker who have progressive metastatic disease that has no satisfactory alternative treatment. Because this tissue-agnostic drug has demonstrated durable responses and safety in this patient population, larotrectinib represents an innovative milestone in precision medicine and in oncology drug development.
References
- Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov. 2015;5:25-34.
- Lange AM, Lo HW. Inhibiting TRK proteins in clinical cancer therapy. Cancers (Basel). 2018;10:105.
- Amatu A, Sartore-Bianchi A, Siena S. NTRK gene fusions as novel targets of cancer therapy across multiple tumour types. ESMO Open. 2016;1:e000023.
- US Food and Drug Administration. FDA approves an oncology drug that targets a key genetic driver of cancer, rather than a specific type of tumor. November 26, 2018. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm626710.htm. Accessed January 13, 2019.
- Vitrakvi (larotrectinib) capsules/oral solution [prescribing information]. Stamford, CT: Loxo Oncology; November 2018.
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in TRK fusion–positive cancers in adults and children. N Engl J Med. 2018;378:731-739.