Lung cancer is the leading cause of death from cancer worldwide, estimated to be responsible for nearly 1 in 5 cancer deaths in 2012 (1.59 million deaths, 19.4% of total cancer deaths).1 In the United States, lung cancer is the second most frequently diagnosed cancer, with an estimated 224,390 new cases in 2016, representing the leading cause of cancer death in Americans.2,3
The 4 major histologic types of lung cancer are squamous-cell carcinoma, adenocarcinoma, large-cell carcinoma, and small-cell undifferentiated carcinoma, which account for more than 90% of lung cancer cases in the United States; 85% of these cases are classified as non–small-cell lung cancer (NSCLC), with squamous-cell NSCLC (SQ-NSCLC) comprising approximately 30%.4 Approximately 80% of all lung cancer cases in men and 90% of cases in women are associated with smoking, with >90% of cases of SQ-NSCLC attributed to cigarette smoking.4,5
Although the 5-year survival rate for localized lung cancer is 54%, only a small proportion (approximately 15%) of lung cancers are diagnosed at this early stage.6 Among all cases of lung cancer that were diagnosed between 2003 and 2009 in the United States, the 1-year relative survival rate was 43%, with a disappointing 5-year overall survival (OS) rate of 17%.6 Moreover, lung cancer is the third most expensive cancer in the United States, with a total estimated annual cost of $10.3 billion.7
Until recently, histologic subtyping of NSCLC was not considered to be clinically important, because different treatment options did not exist for the various subtypes of NSCLC. However, there is now evidence that the classification of NSCLC into histologic subtypes is critical for the selection of appropriate systemic therapy, from the point of view of treatment efficacy as well as for the prevention of toxicity.8
If pathologic findings are missed, especially when differentiating between squamous and nonsquamous histologies, it is difficult to select the most appropriate treatment. Distinguishing between squamous and nonsquamous histologies is typically achieved through preoperative transbronchial biopsy or via computed tomography–guided fine-needle biopsy or the intraoperative collection of a sample for cytologic and immunohistochemical analyses, with acceptable concordance rates of 62% to 98% between methods.8
In recent years, an increasing frequency of peripheral SQ-NSCLC has been reported.9 Pathologic diagnosis can be made through the examination of biopsy specimens with light microscopy and immunohistochemistry.9
Chemotherapy is currently the standard of care for nononcogene-driven advanced NSCLC; however, patients typically undergo multiple lines of chemotherapy as their disease progresses. Treatments have improved in recent years, but limited benefits are typically seen, especially in patients receiving later-line chemotherapy, with low response rates, short response duration, frequent relapse, and poor survival.10
Furthermore, only a small proportion of patients derive benefit from later-line therapy, with most patients experiencing deteriorating quality of life and significant toxicities. Adjuvant chemotherapy after surgical resection provides only modest improvements in cure rates, with disease recurrence occurring in a substantial proportion of patients.11 This represents a significant unmet clinical need in the treatment of patients with NSCLC, which has triggered research into novel targeted therapies based on histologic findings.
Although targeted therapies have provided improvements in outcomes, these treatments only offer a clear benefit in subsets of tumors harboring the appropriate genomic alteration (ie, mutation, amplification, or translocation). Most of these genomic abnormalities are susceptible to therapeutic intervention and are detected in patients with adenocarcinoma, who are predominantly never-smokers. Although alterations in the genome of other histologic subtypes are known (including squamous-cell histology), specific agents targeting some of these alterations have yet to be developed. Thus, the management of these histologic subtypes represents an ongoing challenge.
In SQ-NSCLC, genomic alterations have not been comprehensively characterized, and no molecularly targeted therapies have been specifically developed for the treatment of this disease until recently. However, molecular genotyping has recently led to the development of targeted agents for mutations that are prevalent in SQ-NSCLC.
This publication highlights the safety and efficacy of newer cytotoxic and targeted agents for the treatment of advanced SQ-NSCLC, with a special focus on the mechanism of action, safety, and efficacy of the novel recombinant human immunoglobulin G1 anti–epidermal growth factor receptor (EGFR) monoclonal antibody necitumumab (Portrazza). Necitumumab was approved by the US Food and Drug Administration (FDA) in November 2015 for use in combination with gemcitabine (Gemzar) and cisplatin (Platinol), as the first biologic therapy for the first-line treatment of patients with metastatic SQ-NSCLC.12
Table 1 outlines the currently available therapies for squamous and nonsquamous NSCLC, which fall into 4 general categories—chemotherapy (primarily platinum-based doublets), anti-EGFR agents, antiangiogenesis agents, and immune checkpoint inhibitors.
Treatment Options for NSCLC
Cytotoxic Chemotherapy
Activating mutations in EGFR and anaplastic lymphoma kinase (ALK) fusions are typically absent in SQ-NSCLC,13 and targeted agents developed for lung adenocarcinoma are largely ineffective against squamous-cell lung cancer. The occasional detection of EGFR and ALK mutations in samples from patients diagnosed with SQ-NSCLC is a result of challenges with the diagnosis of adenosquamous carcinoma and adenocarcinoma, which can be largely resolved by comprehensive pathologic assessment incorporating immunohistochemical markers.13
Cytotoxic chemotherapy for NSCLC has reached a therapeutic plateau: equivalent survival has been demonstrated with 4 different platinum-based doublet chemotherapies, with outcomes not analyzed by histology.14 However, the results of a large phase 3 clinical trial of cisplatin plus pemetrexed (Alimta) versus cisplatin plus gemcitabine indicated a difference in outcomes based on histology, with patients with squamous-cell histology exhibiting a relative survival benefit from treatment with cisplatin plus gemcitabine versus cisplatin plus pemetrexed.15
Moreover, a retrospective subanalysis of a phase 3 trial revealed inferior survival in patients with SQ-NSCLC who were receiving second-line pemetrexed therapy compared with docetaxel (Taxotere),16 and another phase 3 clinical trial showed no benefit from pemetrexed maintenance in the subset population of patients with SQ-NSCLC.17 Based on these results, pemetrexed has been approved by the FDA for the treatment and maintenance therapy of patients with locally advanced or metastatic non–squamous-cell NSCLC; however, it is not recommended for the treatment of patients with SQ-NSCLC.18
Recently, a large phase 3 trial comparing carboplatin (Paraplatin) plus paclitaxel (Taxol) with carboplatin plus nab-paclitaxel (Abraxane) in patients with stage IIIB/IV NSCLC also showed a difference in efficacy based on histology.19 The nab-paclitaxel arm showed an improved overall response rate (the primary end point of the trial) compared with the paclitaxel arm, but this benefit was limited to the SQ-NSCLC subset, which exhibited a 41% radiologic response in the nab-paclitaxel arm compared with 24% in the solvent-based paclitaxel arm. Compared with the paclitaxel group, the nab-paclitaxel group also exhibited a numerically higher, but not statistically significant median OS in SQ-NSCLC.19
Antiangiogenesis Agents
Bevacizumab (Avastin), a vascular endothelial growth factor (VEGF) inhibitor, has shown efficacy in patients with NSCLC.20 Bevacizumab in combination with carboplatin and paclitaxel improved overall response and time to progression in patients with advanced or recurrent NSCLC. However, because major hemoptysis was associated with squamous-cell histology, tumor necrosis and cavitation, and disease location close to major blood vessels, bevacizumab is not recommended for the treatment of patients with SQ-NSCLC.20
Ramucirumab (Cyramza), a VEGF receptor 2 inhibitor, received an expanded approval by the FDA on December 12, 2014, as second-line therapy for metastatic NSCLC.21,22 The approval was based largely on results from the REVEL trial, which compared ramucirumab plus docetaxel to docetaxel plus placebo in patients whose disease progressed while receiving platinum-based chemotherapy.23 The median OS was 10.5 months in the ramucirumab plus docetaxel arm compared with 9.1 months in the docetaxel plus placebo arm (hazard ratio [HR], 0.86; 95% confidence interval [CI], 0.75-0.98; P = .02). The median progression-free survival (PFS) was also superior in the ramucirumab plus docetaxel arm compared with the docetaxel plus placebo arm (4.5 months vs 3 months; P <.0001).23
Patients with SQ-NSCLC made up approximately 25% of the trial population and showed a numeric, but not statistically significant, improvement in median OS in the ramucirumab plus docetaxel arm (9.5 months vs 8.2 months in the docetaxel plus placebo arm; HR, 0.88; 95% CI, 0.69-1.13).23 (In 2015, ramucirumab received a new indication for the treatment of patients with metastatic colorectal cancer that is progressing with other treatments.24)
Immunotherapy
A recent advance in targeted therapy for SQ-NSCLC involves immune checkpoint inhibitors. Tumors attempt to escape immune surveillance and detection by expressing programmed death ligand-1 (PD-L1), which interacts with the PD-1 receptor on T-cells. This interaction leads to the downregulation of T-cell activity and suppression of the host antitumor cellular response.25
Immune checkpoint inhibitors are being developed to interfere with the PD-1 and PD-L1 interaction, with the goal of preventing the downregulation of antitumor T-cell activity. Two immune checkpoint inhibitors, nivolumab (Opdivo) and pembrolizumab (Keytruda), have received FDA approval for NSCLC.
The PD-L1 inhibitor pembrolizumab was approved in 2015 for use in NSCLC across squamous and nonsquamous histologies in patients whose disease progressed while or after receiving platinum-containing chemotherapy.26 The other anti–PD-1 monoclonal antibody, nivolumab, received an expanded indication for metastatic SQ-NSCLC on March 4, 2015.27
> Immune checkpoint inhibition with monoclonal antibodies against cytotoxic T-lymphocyte–associated protein 4 has also been a topic of research in NSCLC. Several antibodies are being evaluated for SQ-NSCLC.28,29EGFR Inhibitors in NSCLC
In patients with an EGFR-activating gene mutation, the EGFR tyrosine kinase inhibitors (TKIs) gefitinib (Iressa), erlotinib (Tarceva), afatinib (Gilotrif), cetuximab (Erbitux), and crizotinib (Xalkori) can improve PFS and OS compared with cytotoxic chemotherapy.30-39 EGFR-activating genetic mutations are found in approximately 20% of patients with adenocarcinomas40; the prevalence in patients with squamous-cell cancers is considerably lower.41
A retrospective study of patients with advanced SQ-NSCLC who received erlotinib showed that 16 of 92 (17%) patients achieved a partial response and 9 of 92 (10%) patients had stable disease.42 Moreover, the SATURN trial examined the efficacy of erlotinib as a maintenance treatment in advanced NSCLC; the results showed that erlotinib prolonged PFS compared with placebo in EGFR-mutation–positive and EGFR-mutation–negative tumors, but the difference in PFS did not reach statistical significance in the squamous-cell subset population.43 The TAILOR trial comparing erlotinib with docetaxel as second-line treatment in patients with wild-type EGFR stage IV NSCLC showed a median OS of 8.2 months for the docetaxel arm versus 5.4 months for erlotinib; the results trended in a similar direction for the squamous-cell subset.44,45
The LUX-Lung 8 trial was a prospective, open-label phase 3 trial comparing 2 EGFR TKIs (afatinib and erlotinib) in patients with relapsed or refractory stage IIIB/IV SQ-NSCLC who had progressed after at least 4 cycles of platinum-based doublet chemotherapy and who had not received previous EGFR inhibitor therapy.46 The median PFS and disease control rate were higher for afatinib than with erlotinib (2.4 months vs 1.9 months, and 51% vs 40%, respectively). Higher incidences of diarrhea and stomatitis were observed with afatinib.46
Cetuximab, a recombinant human/mouse chimeric monoclonal antibody directed against EGFR, has shown minimal survival benefit when combined with cisplatin and vinorelbine (vs chemotherapy alone) in a subset of patients with SQ-NSCLC (9 months with cetuximab vs 8.2 months with cisplatin plus vinorelbine alone).45,47
Although most patients with EGFR-mutated NSCLC receive an EGFR TKI as first-line therapy, the majority of patients experience relapse and disease progression after approximately 10 to 15 months of treatment.48 This is most likely a result of an EGFR exon 20 T790M mutation, which is detected in approximately 50% to 60% of tumor samples in patients experiencing disease progression while receiving EGFR TKIs.49,50 This suggests a pressing need for new therapeutic options, particularly for novel EGFR inhibitors with durable responses.
A First-in-Class EGFR Inhibitor for SQ-NSCLC: Necitumumab
On November 24, 2015, necitumumab (Portrazza) was approved by the FDA, for use in combination with gemcitabine and cisplatin, for first-line treatment of patients with metastatic SQ-NSCLC.12,51 The recommended dose of necitumumab is 800 mg via intravenous infusion over 60 minutes on days 1 and 8 of each 3-week cycle before the infusion of gemcitabine and cisplatin, with treatment continued until disease progression or unacceptable toxicity. Necitumumab is not indicated for the treatment of nonsquamous NSCLC.12,51Mechanism of Action
Necitumumab is a second-generation recombinant human immunoglobulin G1 monoclonal antibody that binds to EGFR and blocks the binding of EGFR to its ligands. Necitumumab was engineered to bind to EGFR with high affinity (ie, dissociation constant, 0.32 nmol/L) and to block the binding of relevant EGFR ligands.52 Necitumumab binding is believed to induce EGFR internalization and degradation in vitro, and to result in antibody-dependent cellular cytotoxicity against EGFR-expressing NSCLC cell lines. In studies in mouse xenografts, including NSCLC, the addition of necitumumab to gemcitabine plus cisplatin resulted in increased antitumor activity.53
The activation of EGFR—a member of the ErbB family of type I receptor tyrosine kinases—has been implicated in the pathogenesis of several human malignancies. The binding of EGFR ligands to EGFR, including epidermal growth factor, transforming growth factor-α, amphiregulin, and betacellulin, has profound effects on cellular proliferation, apoptosis, cell differentiation, and metastasis. This process occurs through the activation of several critical signaling cascades, including the reticular activating system and mitogen-activated protein kinase, phospholipase C-γ, phosphatidylinositol 3-kinase and protein kinase B, and the signal transducer and activator of transcription 3 pathways. EGFR expression and the EGFR-mediated activation of downstream signaling pathways are related to poor outcomes in several human malignancies. Lung cancer is known to coexpress EGFR and its ligands, setting the stage for autocrine activation of EGFR. Indeed, it has been shown that the coexpression of EGFR and its ligands is a poor prognostic indicator in lung cancer and in other tumors.52
The SQUIRE TriaL
Efficacy Data
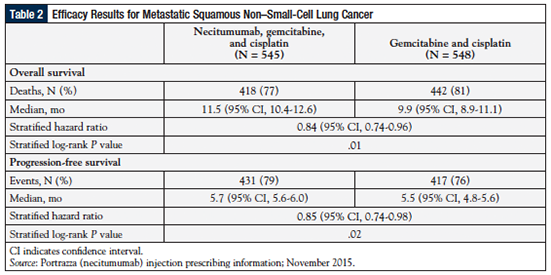
The FDA approved necitumumab based largely on the results of the SQUIRE trial, an open-label, randomized, multicenter, controlled phase 3 trial in which 1093 patients with histologically or cytologically confirmed stage IV SQ-NSCLC were randomly assigned to receive first-line therapy with either necitumumab 800 mg via intravenous infusion on days 1 and 8, plus gemcitabine at 1250 mg/m2 on days 1 and 8, plus cisplatin at 75 mg/m2 on day 1 of each 3-week cycle (N = 545), or to gemcitabine plus cisplatin alone (N = 548). The patients’ median age was 62 years, 92% were smokers, and 90% had metastatic disease in at least 2 sites.54
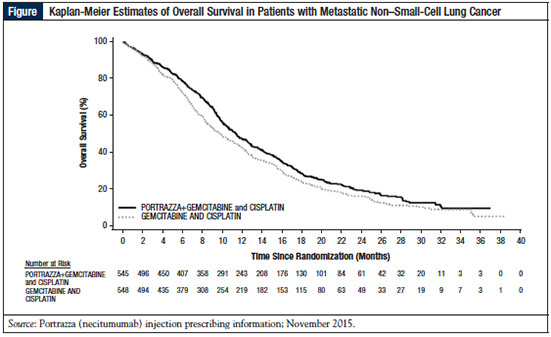
As shown in Table 2 and in the Figure, the median OS (the primary end point) was 11.5 months (95% CI, 10.4-12.6) in the necitumumab group versus 9.9 months (95% CI, 8.9-11.1) in the control group (HR, 0.84; P = .01).51 The median PFS was 5.7 months versus 5.5 months in patients receiving necitumumab versus the control group (HR, 0.85; P = .02; Table 2).51 The time to treatment failure was significantly longer (HR, 0.84; P = .006) and the disease control rate was significantly greater in the necitumumab group than in the control group Cochran-Mantel-Haenszel, P = .043).54
The Kaplan-Meier curves for OS show an early separation in favor of therapy with necitumumab plus gemcitabine and cisplatin from approximately 3 months, and that was maintained for the duration of the study (Figure).51,54
Samples of tissue from 982 of 1093 (90%) patients were evaluable by immunohistochemistry for EGFR protein expression level. In 38% of patients, EGFR expression was high (H-score ≥200), and in 62%, EGFR expression was low (H-score <200).
The HR for OS was higher for the necitumumab group than the placebo group in patients whose tumors had high EGFR expression than in those with low EGFR expression (HR, 0.75 vs 0.90, respectively).54 No difference was seen in PFS between the patients in the high and low EGFR expression groups. However, there was no significant difference in HRs for OS and PFS between the high and low EGFR expression groups according to interaction testing. This suggests that a discriminatory H-score threshold of 200 is not predictive of a differential effect by necitumumab.54
Safety Data
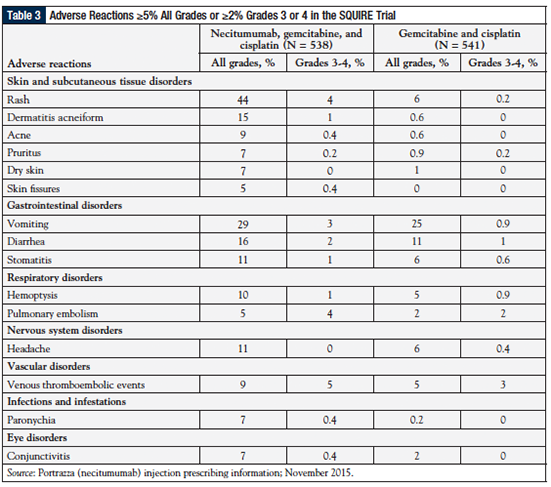
Table 3 shows the adverse events (AEs) of interest in the SQUIRE study (Study 1).51 The most common AEs of any grade occurring at a frequency of ≥2% higher in the necitumumab group than in the chemotherapy group were rash (44% vs 6%, respectively), vomiting (29% vs 25%, respectively), and diarrhea (16% vs 11%, respectively).
The most common grade 3 or 4 AEs occurring at a ≥2% higher rate in the necitumumab group than in the control group were venous thromboembolic events (5%, including pulmonary embolism vs 3%, respectively), rash (4% vs 0.2%, respectively), and vomiting (3% vs 0.9%, respectively). Grade 3 or 4 electrolyte abnormalities observed with necitumumab included hypomagnesemia (20% vs 7% of the control group), hypophosphatemia (8% vs 6%, respectively), hypocalcemia (6% vs 2%, respectively), and hypokalemia (5% vs 3%, respectively). Death attributed to cardiovascular events or sudden death was reported in 3% of the necitumumab group.51,54
AEs leading to the discontinuation of at least 1 study drug were reported by 31% of the patients in the necitumumab group and by 25% of those in the chemotherapy control group. The most common reasons for treatment discontinuation in both groups were neutropenia and thrombocytopenia.54
These findings show that the addition of necitumumab to gemcitabine plus cisplatin as first-line therapy is associated with statistically significant improvements in OS and PFS in patients with advanced SQ-NSCLC, with an acceptable safety profile.54
Several subgroup analyses of the SQUIRE trial have been conducted. For example, a subgroup analysis by performance status of patients treated with necitumumab and gemcitabine plus cisplatin or with gemcitabine plus cisplatin alone showed that the HR for OS was significantly better in the necitumumab group versus chemotherapy alone for the performance status 0-1 group and numerically superior in the performance status 2 group (HR, 0.85; P = .03 in the performance status 0-1 group and HR, 0.78; P = .28 in the performance status 2 group).55
Similarly, the HR for PFS was significantly better in the necitumumab group versus in the cohort receiving chemotherapy alone for the performance status 0-1 group and numerically superior in the performance status 2 group (HR, 0.86; P = .035 in the performance status 0-1 group and HR, 0.79; P = .29 in the performance status 2 group). To summarize, a consistent treatment effect in favor of necitumumab and gemcitabine plus cisplatin for OS and PFS was observed across the performance status 0-1 and performance status 2 subgroups, with no evidence of an increased safety risk in the performance status 2 subgroup.55
A retrospective analysis of the SQUIRE study evaluated the safety and efficacy of single-agent necitumumab continuation or maintenance therapy after the completion of chemotherapy.56 Patients who were alive and free of progression after the completion of chemotherapy demonstrated a consistent treatment effect in favor of continuation necitumumab therapy, with no unexpected increase in AEs.56
The Search for a Molecular Biomarker
The SQUIRE study demonstrated a small, but statistically significant survival benefit in patients treated with necitumumab in combination with cisplatin and gemcitabine compared with those receiving chemotherapy alone, but the identification of a predictive biomarker for treatment outcome is still needed to select the patients who will experience the greatest benefit from the targeted treatment.53
Moreover, the phase 3 INSPIRE study showed that the addition of necitumumab to pemetrexed and cisplatin did not increase the survival of treatment-naive patients with stage IV nonsquamous NSCLC.57 Future studies should identify potentially useful biomarkers to identify the best candidates for necitumumab when combined with standard chemotherapy.
Value-Based Pricing
Value-based care in oncology has become an important issue in view of the rising costs of many new therapeutic agents. Because the SQUIRE trial demonstrated that adding necitumumab to chemotherapy for patients with advanced metastatic SQ-NSCLC produced a median OS benefit of 1.6 months, a study was undertaken to determine the incremental ost-effectiveness ratio (ICER) of standard chemotherapy with and without necitumumab in the first-line treatment of patients with metastatic SQ-NSCLC.58
In the base-care analysis, the addition of necitumumab to chemotherapy produced an incremental survival benefit of 0.15 life-years and 0.11 quality-adjusted life-years (QALYs). The probabilistic sensitivity analyses established that when the cost of necitumumab was <$563 per cycle, there was 90% confidence that the ICER would be <$100,000 per QALY; when the cost increased to $1309 per cycle, there was 90% confidence that the ICER would be <$200,000 per QALY; and when the cost was >$6628 per cycle, there was 99% confidence that the ICER would be >$500,000 per QALY.58 These findings provide a framework for establishing value-based pricing for necitumumab, pointing to the need to evaluate the cost-effectiveness of other oncology drugs entering the US marketplace.
Conclusion
Over the past 2 decades, progress in the treatment of patients with metastatic SQ-NSCLC has been limited, with cytotoxic chemotherapy having reached a virtual therapeutic plateau. Recently, antiangiogenesis agents and immune checkpoint inhibitors have shown promise in improving clinical outcomes in this patient population. Moreover, EGFR has been shown to be involved in tumor progression and invasion in a number of solid tumors, and it has become a favored therapeutic target in NSCLC. Strategies to block the EGFR pathway have been focused on the development of small-molecule TKIs and anti-EGFR monoclonal antibodies.
Now, for the first time, a fully human immunoglobulin G1 monoclonal antibody targeting EGFR, necitumumab (when used first line in combination with standard chemotherapy) has been shown to increase OS in chemotherapy-naïve patients with metastatic NSCLC and confirmed squamous-cell histology. These results have led to the FDA’s approval of the combination of necitumumab plus chemotherapy for this indication. A molecular biomarker is being sought that would predict which patients with SQ-NSCLC, as well as which patients with NSCLC and nonsquamous histology, would most benefit from the addition of necitumumab to standard chemotherapy.
References
- World Health Organization, International Agency for Research on Cancer. GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. http://globocan.iarc.fr/Default.aspx. Accessed January 12, 2016.
- Henley SJ, Richards TB, Underwood JM, et al. Lung cancer incidence trends among men and women—United States, 2005-2009. MMWR Morb Mortal Wkly Rep. 2014;63:1-5.
- American Cancer Society. Cancer Facts & Figures 2016. www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016. Accessed January 26, 2016.
- Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the future. J Clin Oncol. 2005;23:584-594.
- Barbone F, Bovenzi M, Cavallieri, et al. Cigarette smoking and histologic type of lung cancer in men. Chest. 1997;112:1474-1479.
- American Cancer Society. Cancer Facts & Figures 2014. www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed January 29, 2016.
- Hardison BL. The financial burden of cancer. NCI Benchmarks. April 23, 2010. http://benchmarks.cancer.gov/2010/04/the-financial-burden-of-cancer/. Accessed January 29, 2016.
- Yamagishi T, Shimizu K, Ochi N, et al. Histological comparison between preoperative and surgical specimens of non-small cell lung cancer for distinguishing between “squamous” and “non-squamous” cell carcinoma. Diagn Pathol. 2014;9:103.
- Niederhuber JE, Armitage JO, Doroshow JH, et al. In: Abeloff’s Clinical Oncology. 5th ed. Philadelphia, PA: Elsevier Saunders: 1404-1412.
- Scagliotti GV, Bironzo P, Vanstenkiste JF. Addressing the unmet need in lung cancer: the potential of immune-oncology. Cancer Treat Rev. 2015;41:465-475.
- Pakkala S, Ramalingan SS. Adjuvant therapy for nonsmall cell lung cancer: recent advances and future perspectives. Curr Opin Oncol. 2016;28:150-158.
- US Food and Drug Administration. FDA approves Portrazza to treat advanced squamous non-small cell lung cancer. Press release. November 24, 2015. Updated November 25, 2015. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm474131.htm. Accessed February 1, 2016.
- Rekhtman N, Paik PK, Arcila ME, et al. Clarifying the spectrum of driver oncogene mutations in biomarker-verified squamous carcinoma of lung: lack of EGFR/KRAS and presence of PIK3CA/AKT1 mutations. Clin Cancer Res. 2012;18:1167-1176.
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92-98.
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543-3551.
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589-1597.
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432-1440.
- Alimta (pemetrexed) for injection [prescribing information]. Indianapolis, IN: Eli Lilly and Company; February 2015.
- Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30:2055-2062.
- Johnson J, Fehrenbacher L, Novotny EF, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184-2191.
- Cyramza (ramucirumab) injection [prescribing information]. Indianapolis, IN: Eli Lilly; April 2015.
- US Food and Drug Administration. FDA expands approved use of Cyramza to treat aggressive non-small cell lung cancer. Press release. December 12, 2014. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm426720.htm. Accessed February 1, 2016.
- Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665-673.
- US Food and Drug Administration. Ramucirumab mCRC. Press release. April 24, 2015. www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm444496.htm. Accessed February 1, 2016.
- Errico A. Immunotherapy: PD-1-PD-L1 axis: efficient checkpoint blockade against cancer. Nat Rev Clin Oncol. 2015;12:63.
- Keytruda (pembrolizumab) injection [package insert]. Whitehouse Station, NJ: Merck; December 2015.
- US Food and Drug Administration. FDA expands approved use of Opdivo to treat lung cancer. Press release. March 4, 2015. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm436534.htm. Accessed February 1, 2016.
- Tomasini P, Khobta N, Greillier L, Barlesi F. Ipilimumab: its potential in non-small cell lung cancer. Ther Adv Med Oncol. 2012;4:43-50.
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046-2054.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121-128.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380-2388.
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735-742.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239-246.
- Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122-1128.
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naive non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 2013;24:54-59.
- Gao G, Ren S, Li A, et al. Epidermal growth factor receptor-tyrosine kinase inhibitor therapy is effective as first-line treatment of advanced non-small-cell lung cancer with mutated EGFR: a meta-analysis from six phase III randomized controlled trials. Int J Cancer. 2012;131:E822-E829.
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327-3334.
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213-222.
- D’Angelo SP, Pietanza MC, Johnson ML, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol. 2011;29:2066-2070.
- López-Malpartida AV, Ludeña MD, Varela G, García Pichel J. Differential ErbB receptor expression and intracellular signaling activity in lung adenocarcinomas and squamous cell carcinomas. Lung Cancer. 2009;65:25-33.
- Tseng JS, Yang TY, Chen KC, et al. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77:128-133.
- Cappuzzo F, Ciuleanu T, Stelmakh L, et al. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521-529.
- Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14:981-988.
- Derman BA, Mileham KF, Bonomi PD, et al. Treatment of advanced squamous cell carcinoma of the lung: a review. Transl Lung Cancer Res. 2015;4:524-532.
- Soria JC, Felip E, Cobo M, et al. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16:897-907.
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525-1531.
- Stinchcombe TE. Recent advances in the treatment of non-small cell and small cell lung cancer. F1000Prime Rep. 2014;6:117.
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26.
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240-2247.
- Portrazza (necitumumab) injection [prescribing information]. Indianapolis, IN: Eli Lilly; November 2015.
- Kuenen B, Witteveen PO, Ruijter R, et al. A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies. Clin Cancer Res.010;16:1915-1923.
- Greillier L, Tomasini P, Barlesi F. Necitumumab for non-small cell lung cancer. Expert Opin Biol Ther. 2015;15:1231-1239.
- Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763-774.
- Socinski MA, Luft A, Szczesna A, et al. Subgroup analyses by performance status in the phase III SQUIRE study: first-line necitumumab plus gemcitabine-cisplatin (GC) vs. GC in squamous non-small cell lung cancer. J Clin Oncol. 2015;33(suppl):Abstract e19023.
- Ciuleanu TE, Socinski MA, Obasaju CK, et al. Safety and efficacy of necitumumab continuation therapy: subgroup analysis of phase 3 SQUIRE study. J Clin Oncol. 2015;33(suppl):Abstract e19024.
- Paz-Ares L, Mezger J, Ciuleanu TE, et al. Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol. 2015;16:328-337.
- Goldstein DA, Chen Q, Ayer T, et al. Necitumumab in metastatic squamous cell lung cancer: establishing a value-based cost. J Clin Oncol. 2015;33(suppl):Abstract 6505.
Pharmacist’s Perspective
Necitumumab: A New Biologic Active in Metastatic Squamous Non–Small-Cell Lung Cancer
Robert J. Ignoffo, PharmD, FASHP, FCSHPClinical Professor Emeritus, University of California, San Francisco; Professor of Pharmacy, College of Pharmacy, Touro University—California, Mare Island, Vallejo, CA
On November 24, 2015, necitumumab (Portrazza) was approved for the treatment of stage IV squamous non–small-cell lung cancer (NSCLC). Necitumumab is the first biologic to be approved by the US Food and Drug Administration (FDA) for the first-line treatment of patients with squamous NSCLC, for use in combination with gemcitabine (Gemzar) and cisplatin (Platinol).
Mutations in epidermal growth factor receptor (EGFR) and other squamous-cell driver mutations make squamous-cell carcinoma very sensitive to drugs that inhibit such pathways. Necitumumab is a second-generation recombinant monoclonal antibody that competes with the EGFR ligand for binding to the EGFR receptor, preventing downstream activation and signaling.
The FDA approval of necitumumab was based on the recent large phase 3 SQUIRE trial, which randomized 1093 previously untreated patients to cisplatin and gemcitabine with or without necitumumab. The median overall survival (OS) was 11.5 months with the addition of necitumumab to the 2 drugs, showing a clinically significant (albeit modest) improvement of 1.6 months over the 2-drug arm.1 Of note, EGFR tumor expression did not impart prognostic or predictive responses of benefit in this trial.1
As can be expected, grade 3 or 4 toxicities were more prevalent in the 3-drug arm.1 The toxicities that were ≥15% and ≥2% higher with the addition of necitumumab than with gemcitabine and cisplatin alone included rash (44% vs 6%, respectively), vomiting (29% vs 25%), diarrhea (16% vs 11%), and dermatitis acneiform (15% vs 0.6%).1 Other studies with EGFR inhibitors have resulted in mixed results in OS.2-4
Until the recent advent of necitumumab, doublet platinum-based chemotherapy has been the standard treatment for this patient population. Squamous-cell carcinoma occurs in approximately 30% of patients with lung cancer and is exceedingly resistant to systemic chemotherapy, often spreading to hilar and mediastinal lymph nodes, bone, liver, kidneys, adrenal glands, and the gastrointestinal tract.5 According to the National Cancer Institute’s Surveillance, Epidemiology, and End Results program, the 5-year survival rate of patients with metastatic squamous NSCLC is <5%,5 indicating an area that requires improved treatment options. In a recent commentary in Lancet Oncology, Schiller states, “Many factors are involved in the decision of whether a new regimen should become a new first-line treatment. These factors include the size of the benefit, adverse effects, and, unavoidably, cost.”6 Accordingly, these factors should be considered when choosing a treatment regimen.
Although the SQUIRE trial shows only modest benefit in OS with the addition of necitumumab, it is clear that the EGFR pathway is important in the pathogenesis of squamous-cell carcinoma; hence the use of an EGFR inhibitor, such as necitumumab, offers an important advantage. In addition, the toxicity profile for necitumumab is certainly acceptable, although it can be problematic for some patients.
The availability of necitumumab plus cisplatin and gemcitabine as first-line treatment for patients with metastatic squamous-cell carcinoma provides patients with a new treatment option, especially for those with the EGFR mutation. Necitumumab certainly improves the median OS, and should certainly be offered to patients as a first-line therapy.1
As for cost, most biologics are exceedingly expensive today, and necitumumab is no exception, with a cost of approximately $11,400 monthly.7 However, it is important to note that the manufacturer of the drug offers a medication assistance program to qualified patients, in which the patient’s copay is only $25 per dose, a significant help for patients in financial difficulties who are battling cancer.
The newly discovered biologic factors that are driving the growth of squamous-cell carcinoma have encouraged continued research in this area. Another biologic, nivolumab (Opdivo), a programmed death 1 (PD-1) immune checkpoint inhibitor antibody, was approved late last year by the FDA as a second-line treatment for advanced squamous-cell lung cancer. However, PD-1 expression was neither prognostic nor predictive in this study.8
Additional genetic mutations are being discovered that play a role in the proliferation of squamous-cell carcinoma. As a result, the National Cancer Institute has started the Lung-MAP Biomarker-Targeted Second-Line Therapy in Treating Patients with Recurrent Stage IIIB-IV Squamous Cell Lung Cancer (S1400) trial to assess second-line therapy using drugs that target small-cell carcinoma mutations, such as PIK3CA and CCND1.9
Small-cell carcinoma of the lung is receiving more attention as researchers continue to understand the role of biologic factors in the management of this disease. The recent addition of necitumumab, an anti-EGFR monoclonal antibody, as a first-line treatment option for patients with advanced squamous-cell NSCLC provides a clinical benefit for this patient population.
References
- Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16:763-774.
- Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst. 2013;105:595-605.
- Kuan FC, Kuo LT, Chen MC, et al. Overall survival benefits of first-line EGFR tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung cancers: a systematic review and meta-analysis. Br J Cancer. 2015;113:1519-1528.
- Lee CK, Wu YL, Ding PN, et al. Impact of specific epidermal growth factor receptor (EGFR) mutations and clinical characteristics on outcomes after treatment with EGFR tyrosine kinase inhibitors versus chemotherapy in EGFR-mutant lung cancer: a meta-analysis. J Clin Oncol. 2015;33:1958-1965.
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program stat fact sheets: lung and bronchus cancer. http://seer.cancer.gov/statfacts/html/lungb.html. Accessed January 28, 2016.
- Schiller JH. Anti-EGFR monoclonal antibodies in lung cancer treatment. Lancet Oncol. 2015;16:738-739.
- Loftus P. Lilly’s lung-cancer drug Portrazza to cost $11,430 a month. WSJ. December 11, 2015. www.wsj.com/articles/lillys-lung-cancer-drug-portrazza-to-cost-11-430-a-month-1449867424?tesla=y. Accessed January 28, 2016.
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123-135.
- ClinicalTrials.gov. Lung-MAP: S1400 biomarker-targeted second-line therapy in treating patients with recurrent stage IIIB-IV squamous cell lung cancer. https://clinicaltrials.gov/ct2/show/NCT02154490. Updated September 22, 2015. Accessed January 27, 2016.